When your pharmacist hands you a generic pill instead of the brand-name drug your doctor prescribed, you might wonder: Is this really the same thing? It’s not just a label swap. Behind every generic drug you pick up is a rigorous, science-backed system called therapeutic equivalence codes - and it’s the reason you can trust that generic to work just like the brand.
What Therapeutic Equivalence Codes Actually Mean
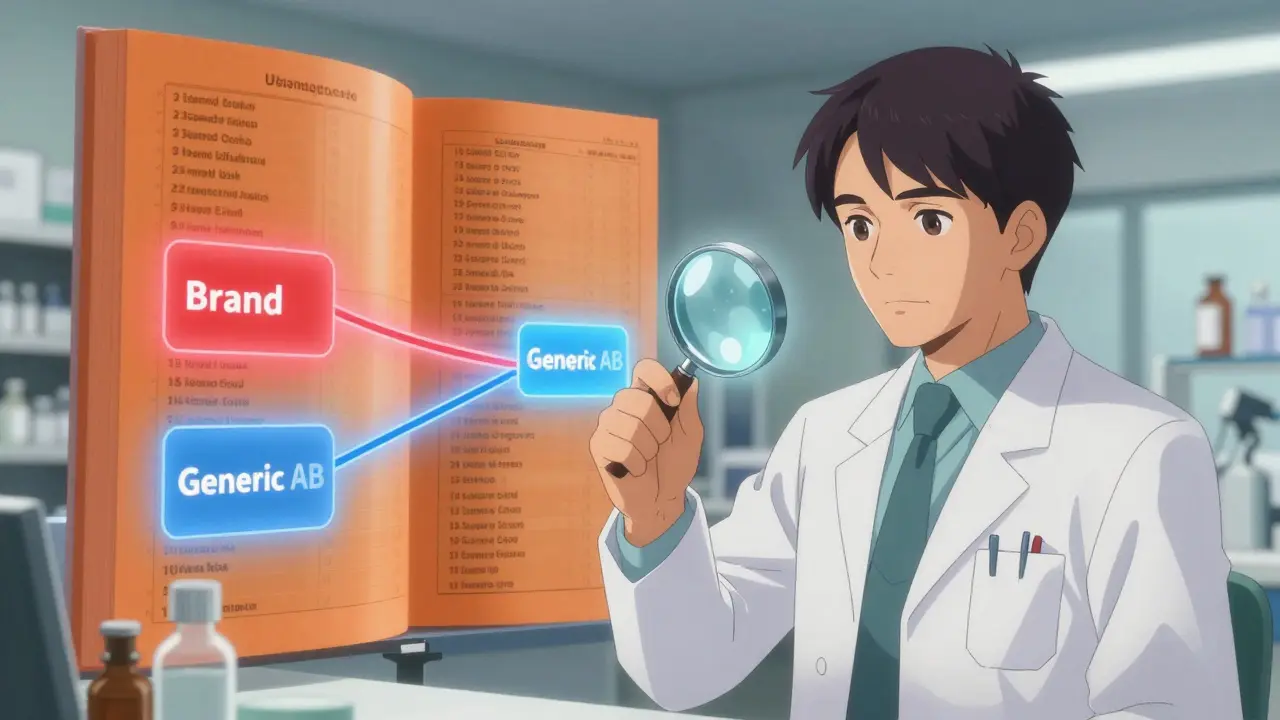
The FDA doesn’t just approve generics because they look similar or cost less. They have to prove they’re therapeutically equivalent - meaning they deliver the same active ingredient, in the same amount, at the same rate, and produce the same clinical effect and safety profile as the brand-name drug. That’s where the Orange Book comes in. Officially called Approved Drug Products with Therapeutic Equivalence Evaluations, the Orange Book has been the FDA’s go-to reference since 1980. Every multisource prescription drug - meaning any drug made by more than one company - gets a code. These codes are short, two-letter labels like AB, BC, or BX. The first letter tells you everything you need to know about substitution. If a drug has an A as its first letter, it means the FDA has confirmed it can be safely swapped for the brand-name version - or for any other generic with the same code. That’s the gold standard. Over 90% of generic drugs in the U.S. carry an ‘A’ rating. That’s why pharmacists can automatically substitute them in 49 states without asking your doctor.Understanding the ‘A’ Codes: AB, AB1, AB2, and So On
Not all ‘A’ ratings are the same. The second letter gives more detail. The most common is AB. This means the generic has passed bioequivalence testing - it releases the drug into your bloodstream at the same rate and amount as the brand. For simple pills like metformin or lisinopril, this is straightforward. The body absorbs them the same way, no matter who made them. But here’s where it gets tricky: some drugs have multiple brand-name versions (called Reference Listed Drugs, or RLDs). If two generics are both equivalent to different brand versions, they can’t always be swapped with each other. That’s why you see codes like AB1, AB2, AB3, and AB4. Each number points to a different reference drug. If your prescription says AB1, your pharmacist can only substitute another AB1 - not an AB2, even if both are labeled as generics. Pharmacists check this every time. In fact, a 2023 survey found that 73% of community pharmacists consult the Orange Book at least once a week to confirm these codes. One wrong substitution could mean a patient gets a drug that doesn’t work the same way - even if both are technically generics.What the ‘B’ Codes Really Tell You
Now, here’s the part that causes confusion: B codes. If a drug has a ‘B’ as its first letter, it means the FDA has not determined it’s therapeutically equivalent. That doesn’t mean it’s unsafe. It means there’s not enough proof yet - or there are complications. Some common ‘B’ codes:- BC - Extended-release products where bioequivalence is hard to prove
- BT - Topical creams or gels with delivery issues
- BN - Inhalers or nebulizers
- BP - Products with known bioequivalence problems
- BX - Not enough data to decide
Why This System Matters for Your Health and Wallet
The U.S. spends about $470 billion on prescription drugs every year. Generics make up 90% of prescriptions but only 23% of that cost. That’s $370 billion saved annually - mostly because of therapeutic equivalence codes. Without the Orange Book and its clear codes, pharmacists couldn’t substitute generics with confidence. Doctors wouldn’t know which ones are interchangeable. Patients might pay more than they need to. The entire system is built on trust - trust that the science is solid, and that the FDA’s evaluations are consistent. And it works. Since the system started, there have been no documented cases of therapeutic failure directly caused by substituting an FDA-approved generic drug. That’s not luck. It’s science.Where the System Falls Short
The TE code system was designed for simple, oral tablets. It’s brilliant for drugs like atorvastatin or amoxicillin. But it struggles with complex products - things like injectables, inhalers, topical creams, and extended-release formulations. For these, traditional bioequivalence studies - which measure drug levels in the blood - don’t always capture what matters. A cream might absorb differently on different skin types. An inhaler might deposit differently in the lungs. A slow-release pill might break down unpredictably in different stomach environments. The FDA admits this. In their 2022 draft guidance, they acknowledged that current methods aren’t enough for complex generics. That’s why the number of ‘B’-rated applications for these products rose 22% between 2018 and 2022. Experts like Dr. Duxin Sun from the University of Michigan warn that we’re relying on outdated tools for modern drugs. The FDA’s goal is to reduce ‘B’ ratings for complex generics by 30% by 2027. That means new testing methods - maybe using real-world data, advanced imaging, or in vitro models - will be needed.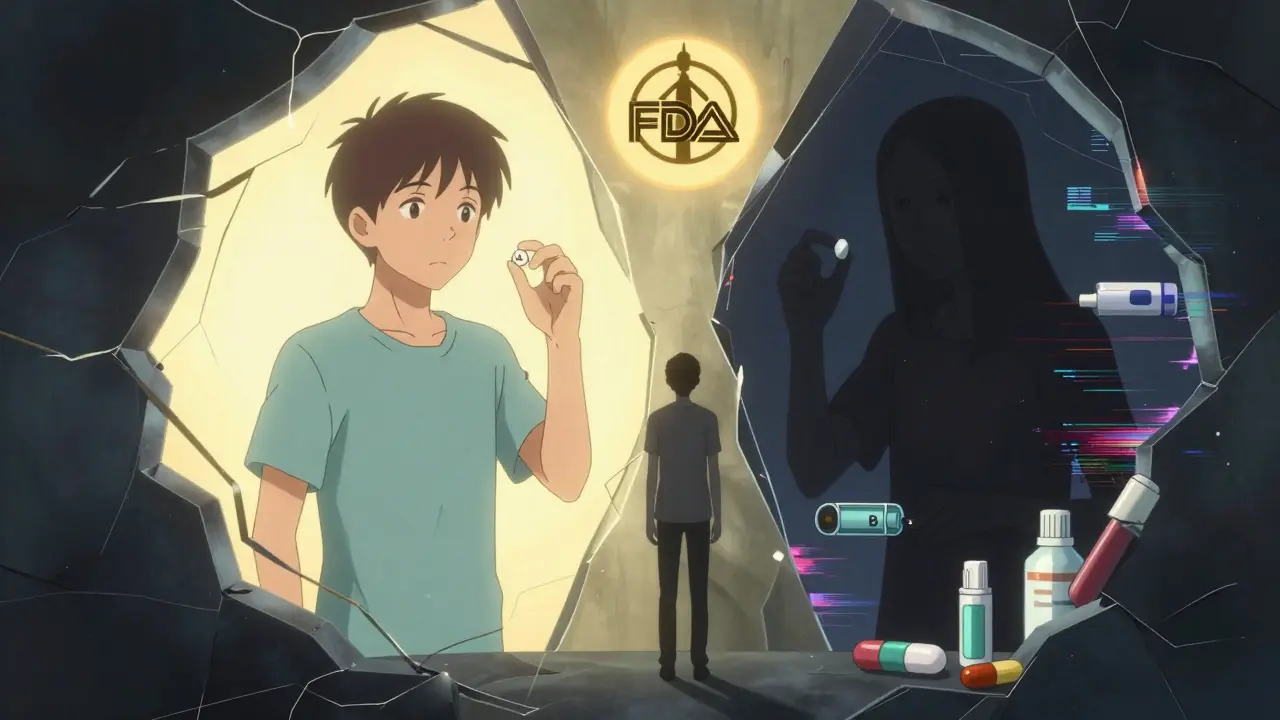
What You Should Do as a Patient
You don’t need to memorize AB1 or BX. But you should know this:- If your prescription says “generic,” and you get a pill that looks different - that’s normal. The color, shape, or imprint doesn’t matter. What matters is the active ingredient and the TE code.
- If your pharmacist says they can’t substitute your drug, ask why. If it’s a ‘B’ code, they’re following the rules. But if they say it’s “not safe,” they might be mistaken.
- Always check the label. The active ingredient should match your brand-name drug exactly.
- If you notice a change in how you feel after switching - fatigue, dizziness, or reduced effectiveness - tell your doctor. It’s rare, but it can happen, especially with complex drugs.
How Pharmacists Use the Orange Book Every Day
Pharmacists don’t guess. They look it up. The FDA’s Orange Book website gets over 1.7 million visits per quarter. That’s doctors, pharmacists, and even insurance companies checking codes before approving a claim. Each time a pharmacist fills a prescription, they check:- Is the generic pharmaceutically equivalent? (Same ingredient, dose, form, route)
- What’s the TE code?
- Is it an ‘A’ code? If yes, substitution is allowed.
- If it’s a ‘B’ code, do state laws require me to notify the prescriber?
What’s Next for Therapeutic Equivalence?
The FDA isn’t resting. They’re updating their methods. They’ve created over 1,850 Product-Specific Guidelines - detailed roadmaps for how to test bioequivalence for specific drugs. These help manufacturers design better generics and get ‘A’ ratings faster. Future changes might include:- Using real-world data from electronic health records to supplement lab studies
- Testing drug absorption in different patient populations (like elderly or obese patients)
- Standardizing testing for inhalers and topical products
Can I ask my pharmacist to switch me to a different generic if my current one isn’t working?
Yes - but only if both generics have the same TE code. For example, if you’re on an AB1 generic and it’s not working, your pharmacist can switch you to another AB1. But they can’t switch you to an AB2, even if it’s the same drug, because it’s linked to a different brand-name version. If you’re having side effects or reduced effectiveness, talk to your doctor. They can prescribe a specific brand or generic by name.
Are all generics just as good as brand-name drugs?
For most drugs - yes. Over 90% of generics are rated ‘A’ by the FDA, meaning they’ve passed strict bioequivalence tests and work the same way. The FDA requires them to deliver the same active ingredient at the same rate and amount. The only exceptions are complex products like inhalers, topical creams, or extended-release pills, which may carry a ‘B’ code. Even then, a ‘B’ doesn’t mean the drug is bad - just that more data is needed.
Why do some generics look different from the brand?
By law, generics can’t look identical to brand-name drugs - that would be trademark infringement. So they can have different colors, shapes, or markings. But the active ingredient, strength, dosage form, and route of administration must be the same. The TE code tells you whether those differences affect how the drug works - not how it looks.
Can I request a brand-name drug even if a generic is available?
Absolutely. Your doctor can write “Dispense as Written” or “Do Not Substitute” on your prescription. Insurance might charge you more, but you have the right to choose. Some patients prefer brand-name drugs for psychological reasons or because they’ve had issues with generics in the past. That’s valid - and your doctor can help you navigate it.
Do over-the-counter (OTC) drugs have therapeutic equivalence codes?
No. The FDA’s therapeutic equivalence system only applies to prescription drugs. OTC medications like ibuprofen or loratadine are regulated under different standards. You can still compare ingredients - for example, store-brand ibuprofen has the same active ingredient as Advil - but there’s no official code system for substitution.







Comments (14)
Kuldipsinh Rathod
December 27, 2025 AT 06:13 AMMan, I never thought about how much science goes into generic pills. I just assumed they were cheap copies. Learning about the AB codes made me feel way more confident switching. Thanks for explaining this so clearly.
Alex Ragen
December 27, 2025 AT 12:30 PMAh, yes-the FDA’s Orange Book: a bastion of bureaucratic precision in an age of pharmaceutical chaos. The ‘A’ rating, a semiotic triumph of regulatory epistemology, affirms not merely equivalence, but the very ontological parity of the generic with its branded progenitor. Yet, one must ask: is bioequivalence truly sufficient when pharmacokinetics are mediated by the capricious whims of human gut flora? The system is elegant-but is it profound?
Lori Anne Franklin
December 28, 2025 AT 01:25 AMok so i just learned that bc means extended release is hard to test?? that makes so much sense now-my uncle’s blood pressure med switched and he felt weird for a week. i thought it was just him, but maybe it was the bc code. thanks for this!!
Ellie Stretshberry
December 29, 2025 AT 18:33 PMmy grandma takes 7 meds and every time she gets a new bottle she freaks out because it looks different. i showed her the active ingredient on the label and told her if it's an A code, it's fine. she still doesn't trust it but at least she stops yelling at the pharmacist now. thanks for making this easier to explain.
Zina Constantin
December 31, 2025 AT 10:38 AMAs someone who grew up in a country where generics were unreliable, seeing how rigorously the U.S. system works gives me hope. This isn’t just about money-it’s about dignity. Everyone deserves safe, affordable medicine. Thank you for highlighting the science behind it.
Jay Ara
January 1, 2026 AT 16:02 PMbro the orange book is like the bible for pharmacists. i work at a pharmacy and we check it like 10 times a day. people get mad when we cant swap their meds but its not us-its the fda rules. just ask for the code. its on the bottle.
SHAKTI BHARDWAJ
January 2, 2026 AT 10:17 AMTHIS SYSTEM IS A SCAM!! I switched to a generic and my anxiety got worse!! The FDA doesn't care about people!! They just want pharma companies to make more money!! B codes are just a cover-up for lazy testing!! I'm going to start a petition!!
Jody Kennedy
January 4, 2026 AT 09:55 AMWow, I had no idea about AB1 vs AB2. I’ve been getting the same generic for years and never realized there were different versions. Now I’m kinda paranoid-should I ask my doc to specify the code next time?
christian ebongue
January 4, 2026 AT 20:57 PMSo… you’re telling me the ‘B’ in BX stands for ‘Bullshit’? Because that’s what it feels like when your inhaler doesn’t work and the pharmacist says ‘it’s the same’. 🤷♂️
jesse chen
January 5, 2026 AT 19:47 PMJust to clarify: BX doesn’t mean it’s unsafe-it means the FDA doesn’t have enough data to say it’s equivalent. That’s not a failure of the system; it’s transparency. The fact that they admit uncertainty is what makes this trustworthy.
Prasanthi Kontemukkala
January 6, 2026 AT 20:42 PMIt’s amazing how much we take for granted. I used to think generics were just cheaper versions, but now I see they’re actually the result of years of research and regulation. Thanks for helping me appreciate the quiet heroes behind my prescription.
Bryan Woods
January 7, 2026 AT 08:55 AMThe system works well for simple oral solids. For complex products, however, the current bioequivalence paradigm is inadequate. Regulatory science must evolve to meet the demands of modern therapeutics.
Ryan Cheng
January 8, 2026 AT 09:58 AMMy cousin’s kid has epilepsy and they switched generics once-seizures came back. Turned out it was a BC-rated drug. They had to go back to brand. Scary stuff. This isn’t just theory-it’s life or death for some people.
wendy parrales fong
January 8, 2026 AT 11:37 AMi think the real hero here is the pharmacist. they’re the ones checking all these codes, answering questions, making sure we don’t get the wrong pill. we should thank them more. they’re like medicine detectives.