When a pharmacist hands you a pill bottle with a different name than what your doctor wrote, you might wonder: is this really the same thing? It’s not just a label change. It’s a legal, scientific, and clinical decision - and pharmacists are the ones who make sure it’s safe.
Why Generic Substitution Matters
Nearly 91% of all prescriptions filled in the U.S. are for generic drugs. That’s over 8.9 billion prescriptions in 2023 alone. Generics save patients and the healthcare system billions - $12.7 billion annually just in verification safeguards. But savings mean nothing if the medicine doesn’t work the same way. That’s where therapeutic equivalence comes in. It’s not enough for a generic to have the same active ingredient. It must deliver the same amount of drug into your bloodstream at the same rate. Otherwise, you could get too little - and the drug won’t work - or too much - and risk side effects.The Orange Book: The Pharmacist’s Bible
The go-to tool for every pharmacist in the U.S. is the FDA’s Approved Drug Products with Therapeutic Equivalence Evaluations, better known as the Orange Book. First published in 1980, it’s updated monthly and lists over 16,500 drug products. Of those, nearly 15,900 are rated as therapeutically equivalent - meaning they’re legally interchangeable. The Orange Book doesn’t just list drugs. It assigns each one a two-letter code. The first letter tells you if it’s equivalent:- A = Therapeutically equivalent to the brand-name drug
- B = Not considered equivalent
What Makes a Generic ‘Equivalent’?
Pharmacists check three layers before swapping a brand for a generic:- Pharmaceutical equivalence - Same active ingredient, same strength, same form (tablet, capsule, injection), same route (oral, topical, etc.).
- Bioequivalence - The generic must release the drug into your blood at nearly the same speed and amount as the brand. The FDA requires the 90% confidence interval for both peak concentration (Cmax) and total exposure (AUC) to fall between 80% and 125% of the brand.
- Therapeutic equivalence - The final call. If the Orange Book says ‘A’, the pharmacist can legally substitute it without harming the patient.
How Pharmacists Actually Do It
It’s not guesswork. Pharmacists follow a strict, repeatable process - usually in under 12 seconds per prescription.- Find the reference listed drug (RLD) in the Orange Book - that’s the brand-name version the generic is based on.
- Confirm the generic has the exact same active ingredient, strength, and dosage form.
- Check the TE rating. Only ‘A’-rated products can be substituted.
- Look for any ‘Do Not Substitute’ notes from the prescriber.
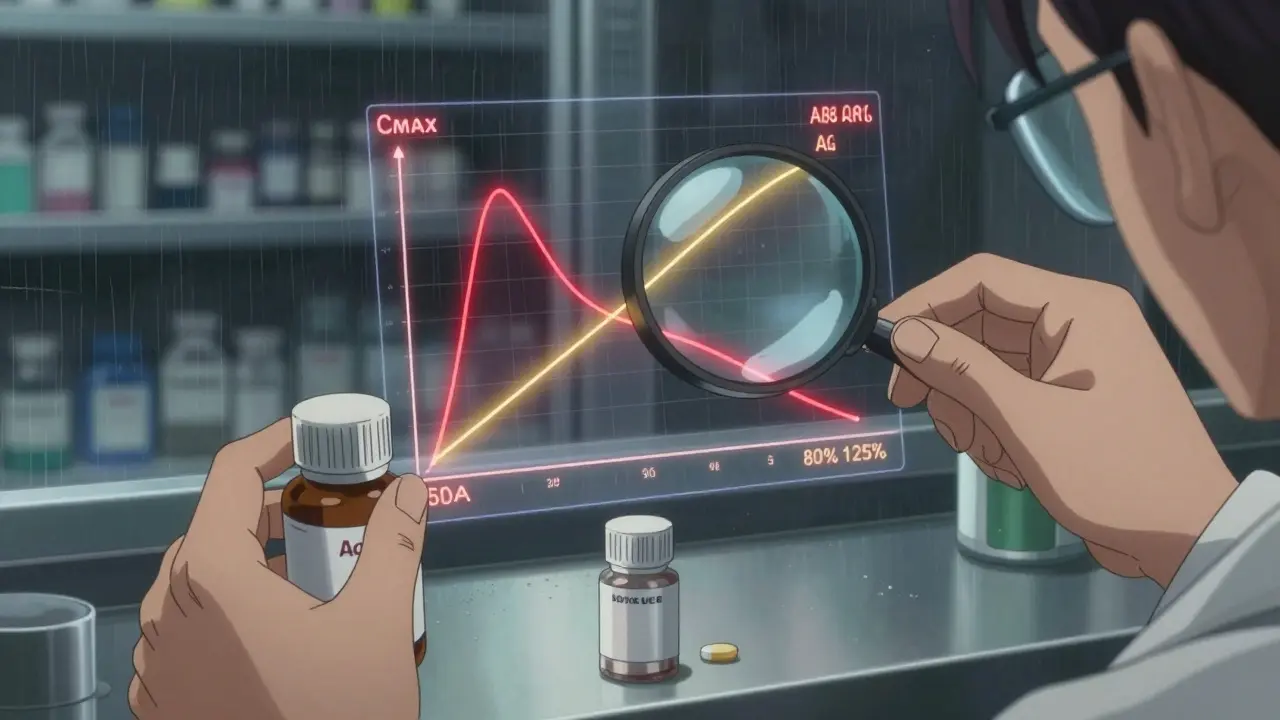
Legal Protection and Real-World Risks
Using the Orange Book isn’t just best practice - it’s the law in 49 states. If a pharmacist substitutes based on an ‘A’ rating and something goes wrong, they’re protected under state substitution laws. The 2019 Texas case State Board of Pharmacy v. Smith shows what happens when they don’t. A pharmacist substituted a generic not listed in the Orange Book. The patient had a bad reaction. The pharmacist lost their license. The American Pharmacists Association and the National Association of Boards of Pharmacy both require Orange Book use in their model guidelines. Training for new hires includes 2-4 hours of hands-on practice. After training, 89.3% of pharmacists correctly identify therapeutic equivalence.Where the System Falls Short
The Orange Book works brilliantly for simple pills and capsules. But it’s less reliable for complex products - inhalers, topical creams, nasal sprays, or injectables where absorption depends on how the drug is delivered, not just how much is in the blood. Dr. Randall Stafford from Stanford pointed out in a 2021 JAMA article that traditional bioequivalence tests don’t capture these nuances. For example, two inhalers might have the same active ingredient and dose, but different propellants or particle sizes could change how much reaches the lungs. The FDA is aware. As of Q2 2024, they’ve issued over 1,850 product-specific guidances for complex generics. They’re also investing $28.5 million through GDUFA III to develop better testing methods - especially for topical corticosteroids and inhaled drugs.Biosimilars: The Next Frontier
The Orange Book was built for small-molecule drugs. It doesn’t handle biologics - complex proteins made from living cells. For those, the FDA uses the Purple Book, which lists biosimilars. But here’s the problem: as of June 2024, only 47 out of 350 approved biosimilars are listed in the Purple Book. Many pharmacists don’t know how to verify them. Unlike generics, biosimilars aren’t automatically interchangeable. Each one requires separate clinical evaluation. This is a growing blind spot. With more biologics entering the market, pharmacists need new tools - and clearer guidance.
The Numbers Don’t Lie
Despite the complexity, the system works. A 2023 study in the Journal of Generic Medicines analyzed over 2,100 bioequivalence studies. 97.8% of generics showed less than a 5% difference in total drug exposure compared to brands. Only 7.2% showed more than a 7% difference in peak concentration - still within FDA limits. The FDA’s 2020 meta-analysis found no meaningful difference in adverse events between brand and generic drugs: 0.78% for brands, 0.81% for generics. The difference? Statistically meaningless. And when pharmacists follow the rules? Substitution errors are extremely rare - just 0.03% according to the American Society of Health-System Pharmacists.What You Can Do
If you’re handed a generic and you’re unsure, ask your pharmacist: “Is this listed as ‘A’ in the Orange Book?” They’re trained to answer. Most will show you the printout or app screen. Don’t assume all generics are the same. If you switch between different generic brands and notice a change - more side effects, less effectiveness - tell your pharmacist. Sometimes, even ‘A’-rated products can have minor formulation differences that affect you personally. And if your doctor writes ‘Dispense as Written’? That’s their choice. The pharmacist must honor it - even if a cheaper, equivalent option exists.What’s Next?
The FDA is working to modernize the Orange Book. By 2026, it will integrate directly with electronic health records, auto-flagging non-equivalent substitutions before the prescription is filled. New digital tools will help pharmacists quickly compare complex products. But the core principle stays the same: equivalence isn’t assumed - it’s verified. And pharmacists are the last line of defense ensuring that what’s written on your script is what’s safe for you to take.Can any generic be swapped for a brand-name drug?
No. Only generics with an ‘A’ rating in the FDA Orange Book are considered therapeutically equivalent and legally interchangeable. Products rated ‘B’ or not listed in the Orange Book cannot be substituted without the prescriber’s approval.
What does ‘AB’ mean in the Orange Book?
‘AB’ means the generic drug is pharmaceutically equivalent and has been shown through human bioequivalence studies to perform the same as the brand-name reference drug. It’s the most common and safest rating for substitution.
Are generic drugs as safe as brand-name drugs?
Yes. FDA data shows the rate of adverse events from generics is statistically identical to brand-name drugs - 0.81% versus 0.78%. The FDA requires generics to meet the same quality, strength, purity, and stability standards as the original.
Why do some people say generics don’t work as well?
Sometimes, it’s not the drug - it’s the patient. Switching between different generic manufacturers can cause slight variations in inactive ingredients, which may affect absorption in sensitive individuals. If you notice a change in how you feel after switching, tell your pharmacist. They can check if a different generic version might be better.
Do pharmacists need special training to verify generics?
Yes. All new pharmacists receive 2-4 hours of formal training on using the Orange Book and verifying therapeutic equivalence. Competency is tested, and 89.3% of trained pharmacists correctly identify equivalent products. This is required by state boards and pharmacy associations.
What if a drug isn’t listed in the Orange Book?
If a generic isn’t listed, pharmacists can’t legally substitute it unless the prescriber allows it. They must use professional judgment, consult FDA guidance on non-listed drugs, and often contact the prescriber before dispensing. These cases make up about 5.7% of generic fills.








Comments (10)
Michael Dillon
December 24, 2025 AT 00:18 AMLook, I get the Orange Book is gospel to pharmacists, but let’s be real - I’ve switched generics three times and each one hit me differently. One made me dizzy, another gave me nausea, the third did nothing. The FDA says it’s all the same, but my body doesn’t care about their confidence intervals. They test blood levels, not how you feel at 3 AM when your brain feels like it’s wrapped in wet cardboard.
Gary Hartung
December 25, 2025 AT 20:15 PMOne must, however, consider the epistemological framework underpinning the so-called 'therapeutic equivalence' paradigm - a construct predicated upon reductionist pharmacokinetic models that utterly fail to account for the phenomenological experience of the patient. The Orange Book, in its bureaucratic myopia, treats the human body as a closed system - a fallacy of the highest order. Bioequivalence is not therapeutic equivalence; it is merely statistical appeasement masquerading as science.
Ben Harris
December 27, 2025 AT 19:30 PMPharmacists don't even read the Orange Book half the time they're just looking at the screen and clicking approve. I've seen it. My cousin works at a chain pharmacy and they get paid bonuses for switching to generics. They don't care if it's AB or BB they just want to hit their quota. And don't even get me started on how they ignore the 'Do Not Substitute' notes because the system auto-fills the generic anyway
Oluwatosin Ayodele
December 29, 2025 AT 06:43 AMMost of you are missing the point. In Nigeria, we don't even have access to the Orange Book. Our generics are imported from India, China, even Pakistan. Some come with no labeling. No TE codes. No FDA oversight. We rely on pharmacists who learned from a 20-year-old manual. If your system works in the U.S., that doesn't mean it's global. What about the rest of the world? You're talking about a luxury.
Jason Jasper
December 30, 2025 AT 15:15 PMI appreciate the thorough breakdown. I’ve had good experiences with generics, but I’ve also had one that gave me weird headaches - switched back to brand, headaches gone. I don’t blame pharmacists - they’re doing their job under pressure. But maybe we need more transparency. Like a little note on the bottle: 'This generic is from Manufacturer X, same as last time?' Just so we know if it changed.
Linda B.
December 31, 2025 AT 10:22 AMDid you know the FDA has been quietly removing some 'A' ratings without public notice? I’ve got a spreadsheet. It started in 2021. They’re clearing out generics that don’t make enough profit. The Orange Book isn’t a safety tool - it’s a corporate filter. And they’re hiding it from you. They don’t want you to know your thyroid med might be a ghost version now.
Christopher King
January 1, 2026 AT 01:29 AMThey say equivalence is verified - but who verifies the verifiers? The FDA outsources bioequivalence testing to private labs that get paid by the drug companies. It’s like letting the fox audit the henhouse. And then they slap an 'AB' on it like it’s a stamp of divine approval. Wake up. This isn’t medicine - it’s a performance art for shareholders.
Bailey Adkison
January 1, 2026 AT 03:01 AMIt’s not about the Orange Book. It’s about the fact that 98% of people don’t know what therapeutic equivalence means. The system works only if patients ask. Most don’t. They just take the pill. And that’s the real failure. Not the science. Not the law. The public’s passive compliance. If you don’t know your meds, you deserve what you get.
Katherine Blumhardt
January 1, 2026 AT 18:19 PMmy pharmacist gave me a generic and i swear it made me feel like a zombie for 3 days 😔 i asked why and she just said 'it's AB rated so it's fine' like that's supposed to comfort me??
sagar patel
January 1, 2026 AT 18:47 PMMy doctor switched me to a generic and my blood pressure spiked. I went back and asked if it was AB rated. She checked the Orange Book. It was. But the manufacturer changed the binder. I switched back to brand. My BP normalized. The system is broken when your body is the test subject.