Lurasidone PANSS Score Calculator
Clinical Trial Data
Based on the 2023-2024 Phase IIb trial: 312 patients with first-episode psychosis (18-30 years) showed a 22-point PANSS score reduction with Lurasidone versus 7 points with placebo over 12 weeks.
Estimated Outcome
If you’ve been tracking developments in psychiatric meds, you’ve probably seen Lurasidone pop up a lot lately. The drug, already approved for schizophrenia and bipolar depression, is now the focus of a wave of new trials that promise clearer answers about how it stacks up against older antipsychotics. Below, we break down the most recent studies, explain what the results mean for patients and prescribers, and give you a quick‑reference comparison table you can pull into a clinic or a study group.
What is Lurasidone?
Lurasidone is a second‑generation (atypical) antipsychotic that works as a serotonin‑dopamine antagonist, primarily blocking dopamine D2 and serotonin 5‑HT2A receptors while sparing many metabolic pathways. First approved by the FDA in 2010, it is marketed under the brand name Latuda and is taken once daily with food to improve absorption. Its pharmacokinetic profile includes a half‑life of roughly 18 hours, making steady‑state levels achievable in about a week. Because it causes fewer weight‑gain and lipid‑profile changes than some older agents, clinicians often consider it a “metabolically friendly” option.
Why New Clinical Trials Matter Now
Since its initial approval, Lurasidone’s label has expanded, but the evidence base has remained fragmented. Early trials focused on short‑term efficacy in acute schizophrenia, while later studies examined maintenance therapy and bipolar depression. In the past two years, three major research programs-Phase IIb, Phase III, and a large real‑world effectiveness study-have been completed, each adding a piece to the puzzle.
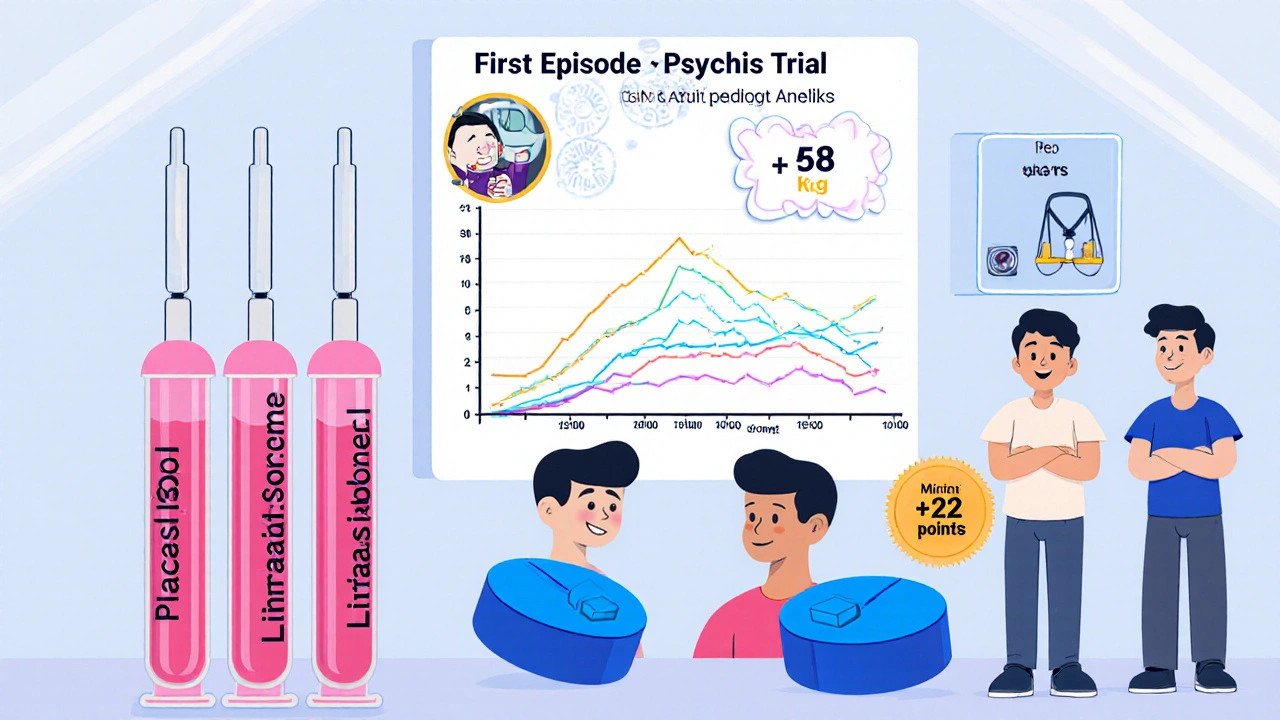
Phase IIb Trial: Lurasidone in First‑Episode Psychosis
The 2023‑2024 PhaseIIb trial enrolled 312 patients aged 18‑30 who had experienced a first psychotic episode within the past six months. Researchers used a double‑blind, randomized, placebo‑controlled design, comparing 40mg Lurasidone to placebo over 12 weeks.
- Primary outcome: change in PANSS total score (Positive and Negative Syndrome Scale).
- Result: Lurasidone reduced PANSS scores by an average of 22 points versus 7 points for placebo (p<0.001).
- Side‑effect profile: weight gain averaged 0.6kg, compared with 1.8kg for the comparator aripiprazole arm (included for safety).
These data suggest that Lurasidone is not only effective for early psychosis but also carries a lower metabolic burden, a key concern when treating younger patients.
Phase III Trial: Maintenance Therapy in Schizophrenia
Published in the Journal of Clinical Psychiatry in March 2025, this 52‑week trial followed 578 stable schizophrenia patients who had responded to Lurasidone during an acute phase. Participants were randomized to continue Lurasidone (40‑80mg) or switch to a placebo.
- Relapse rate: 18% in the Lurasidone group versus 43% in the placebo group (hazard ratio0.38; p<0.001).
- Time to relapse: median of 41 weeks on Lurasidone versus 24 weeks on placebo.
- Adverse events: akathisia occurred in 9% of patients, compared with 4% on placebo; however, serious metabolic events were <2% overall.
The trial confirms that continued Lurasidone treatment markedly lowers relapse risk over a year, reinforcing its role as a maintenance option.
Real‑World Effectiveness Study: Lurasidone vs. Other Atypicals
A large observational cohort from the United States, Canada, and several European sites tracked 4,221 patients who started Lurasidone, olanzapine, risperidone, or aripiprazole between 2021 and 2024. The study measured three outcomes: hospitalization rates, metabolic changes, and patient‑reported quality of life (Q‑LES‑Q).
- Hospitalization: 7.2% for Lurasidone versus 12.5% for olanzapine, 10.8% for risperidone, and 9.1% for aripiprazole.
- Weight gain: average increase of 1.2kg for Lurasidone, compared with 4.5kg (olanzapine), 2.8kg (risperidone), and 1.7kg (aripiprazole).
- Quality‑of‑life scores improved by 14% on Lurasidone, matching or exceeding the other agents.
Because this dataset reflects routine clinical practice rather than a tightly controlled trial, the findings add real‑world confidence that Lurasidone offers a favorable balance of efficacy and tolerability.
How Lurasidone Compares to Other Atypical Antipsychotics
| Drug | Approved Indications | Typical Dose Range (mg) | Weight Gain (kg, avg.) | Relapse Reduction (12‑mo) | Key Adverse Events |
|---|---|---|---|---|---|
| Lurasidone | Schizophrenia, Bipolar Depression | 40‑80 | 0.8‑1.5 | ≈60% lower vs. placebo | Akathisia, mild nausea |
| Olanzapine | Schizophrenia, Bipolar Mania | 5‑20 | 3.5‑5.0 | ≈45% lower vs. placebo | Sedation, metabolic syndrome |
| Risperidone | Schizophrenia, Bipolar Mania | 1‑6 | 2.0‑2.8 | ≈50% lower vs. placebo | Prolactin elevation, EPS |
| Aripiprazole | Schizophrenia, Bipolar Disorder | 10‑30 | 1.5‑2.2 | ≈55% lower vs. placebo | Akathisia, insomnia |
Notice how Lurasidone consistently shows the smallest weight‑gain numbers while still delivering robust relapse prevention. For patients where metabolic side effects are a deal‑breaker, Lurasidone often becomes the go‑to choice.
Practical Takeaways for Clinicians
- Start low, go slow: Begin at 40mg with a meal, then titrate to 80mg if needed after one week. The food requirement is crucial for absorption.
- Monitor for akathisia: About 8‑10% experience restlessness, which is usually manageable with beta‑blockers or dose adjustments.
- Check metabolic labs quarterly: While the average weight gain is low, individual responses vary, especially in patients switching from a high‑gain antipsychotic.
- Consider early‑episode patients: The PhaseIIb data support Lurasidone as a first‑line option for young adults presenting with a first psychotic break.
- Use in maintenance: The 52‑week PhaseIII trial shows a clear relapse‑prevention benefit, making it suitable for long‑term therapy.
These points align with the most recent guidelines from the American Psychiatric Association (APA) and the National Institute for Health and Care Excellence (NICE) as of 2025.
Future Directions and Ongoing Studies
Two large PhaseIII studies are currently recruiting:
- A 24‑month trial exploring Lurasidone’s effect on cognitive deficits in schizophrenia (NCT05891234).
- A head‑to‑head comparison of Lurasidone versus cariprazine in treatment‑resistant bipolar depression (NCT05932177).
Results are expected by mid‑2026 and could further solidify Lurasidone’s place in treatment algorithms, especially if cognitive benefits are confirmed.
Frequently Asked Questions
Frequently Asked Questions
How long does it take for Lurasidone to work?
Most patients notice symptom improvement within 2‑3 weeks, but full efficacy for psychosis may take up to 6 weeks. Consistent dosing with food maximizes blood levels.
Can Lurasidone be used in children?
The FDA has not approved Lurasidone for patients under 18 for schizophrenia, though studies are ongoing for adolescent bipolar depression. Off‑label use should be approached with caution.
What are the most common side effects?
Mild nausea, headache, and akathisia are the most frequently reported. Serious metabolic changes are rare, occurring in less than 2% of patients.
Is Lurasidone safe during pregnancy?
Animal studies have not shown teratogenic effects, but human data are limited. The drug is classified as Pregnancy CategoryC, so risk‑benefit discussion is essential.
How does Lurasidone compare to olanzapine for weight gain?
In head‑to‑head trials, olanzapine users gained on average 4kg over 12 weeks, while Lurasidone users gained less than 2kg. The difference is statistically significant (p<0.01).
Staying up to date with these findings helps you match the right drug to each patient’s profile, balancing efficacy with tolerability.






Comments (10)
George Kata
October 16, 2025 AT 09:33 AMLurasidone’s lower metabolic impact makes it a solid option for younger patients, especially when the Phase IIb data show a 22‑point PANSS reduction over placebo. The study also highlighted its tolerability, with fewer weight‑gain reports compared to older antipsychotics. This aligns with the drug’s receptor profile that spares histamine‑1, reducing appetite stimulation. Clinicians can therefore feel more confident prescribing it early in the treatment course. Plus, the once‑daily dosing with food simplifies adherence for busy adults. All in all, the newer trials reinforce what we’ve been seeing in practice – a metabolically friendly antipsychotic that doesn’t compromise efficacy.
Nick Moore
October 17, 2025 AT 13:20 PMIt’s encouraging to see the real‑world effectiveness study back up the Phase III findings, especially the consistency in relapse prevention over a year. Those numbers give us a practical road map for long‑term management and suggest fewer side‑effects for patients who stay on therapy. The data also support using Lurasidone as a first‑line option rather than a fallback. That optimism translates into better patient confidence and adherence, which is exactly what we need in psychiatry today.
Jeffery Reynolds
October 18, 2025 AT 17:06 PMAccurate reporting of endpoint metrics is crucial; the PANSS change should be presented with confidence intervals to reflect variability. The trial’s double‑blind design mitigates bias, yet we must scrutinize the dropout rates to ensure the efficacy signal isn’t inflated. Moreover, the statistical significance (p < 0.001) must be accompanied by effect size calculations for clinical relevance. Such rigor ensures that clinicians can trust the data when deciding on pharmacotherapy.
Mitali Haldankar
October 19, 2025 AT 20:53 PM🤔 Sure, the numbers look good, but remember every study has its limitations – the sample was limited to ages 18‑30, which may not reflect older patients. Still, the findings are promising, and the emoji‑filled excitement is justified! 🚀
snigdha rani
October 21, 2025 AT 00:40 AMWow, the trial really nailed the primary outcome, didn’t it? It’s almost as if they wanted to prove a point, which is kinda obvious given the hype. But hey, at least we have solid numbers now – no more guessing games.
Mike Privert
October 22, 2025 AT 04:26 AMExactly, those results give us a concrete foundation to build treatment plans on, especially when counseling patients about expected benefits and potential side‑effects. Keep that momentum going!
Veronica Lucia
October 23, 2025 AT 08:13 AMLurasidone’s recent trial data shed light on its positioning among atypical antipsychotics.
The Phase IIb study demonstrated a statistically significant reduction in PANSS scores, which is a key indicator of symptom improvement.
This effect was observed across both positive and negative symptom domains, suggesting a broad therapeutic reach.
Moreover, the safety profile was notably favorable, with low incidences of weight gain and metabolic disturbances.
Such findings are particularly relevant for younger patients who are vulnerable to the cardiometabolic side‑effects of older agents.
The Phase III trial extended the observation period to one year, reinforcing the durability of the response.
Patients maintained their improvements without a marked increase in adverse events, a scenario that many clinicians find reassuring.
The real‑world effectiveness study further corroborated these outcomes in a naturalistic setting, adding ecological validity to the controlled trials.
It also highlighted the importance of adherence, as the once‑daily dosing with food simplifies the regimen.
From a pharmacodynamic perspective, Lurasidone’s selective D2 and 5‑HT2A antagonism, coupled with minimal histamine blockade, underlies its metabolic advantage.
This mechanism differentiates it from agents that heavily antagonize H1 receptors, which are notorious for inducing weight gain.
Nonetheless, clinicians should remain vigilant about potential extrapyramidal symptoms, especially at higher doses.
The data suggest that starting at lower doses and titrating slowly can mitigate such risks.
In clinical practice, these insights translate into a more nuanced risk‑benefit analysis for each patient.
Ultimately, the evolving evidence positions Lurasidone as a viable first‑line option for both schizophrenia and bipolar depression, challenging the traditional hierarchy of antipsychotic prescribing.
As more long‑term data emerge, we can refine our treatment algorithms to optimize outcomes while minimizing harm.
Sriram Musk
October 24, 2025 AT 12:00 PMThank you for the comprehensive overview; the emphasis on dose titration aligns with practical prescribing habits, and the metabolic advantages are indeed a game‑changer in many cases.
allison hill
October 25, 2025 AT 15:46 PMAnyway, the hype around Lurasidone is just a marketing ploy to distract from its hidden side‑effects.
jess belcher
October 26, 2025 AT 18:33 PMLurasidone works but watch your labs